Search Result
Results for "
primary amino group
" in MedChemExpress (MCE) Product Catalog:
11
Biochemical Assay Reagents
2
Isotope-Labeled Compounds
| Cat. No. |
Product Name |
Target |
Research Areas |
Chemical Structure |
-
- HY-W614180
-
|
|
Biochemical Assay Reagents
|
Others
|
|
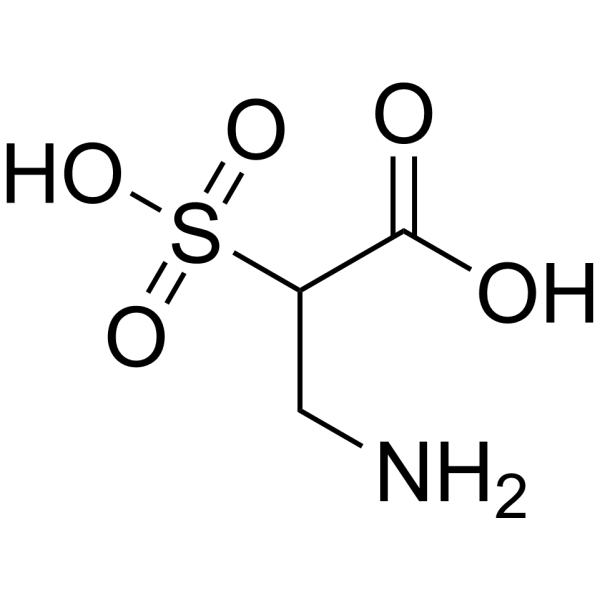
3-Amino-2-sulfopropanoic acid is a short molecule featuring a carboxylic acid, a sulfonate group, and a primary amine. The carboxylic acid and the primary amine are free to link this molecule to larger structures while the sulfonate group provides high water solubility to this aliphatic compound.
|
-
-
- HY-W048673
-
|
|
Amino Acid Derivatives
|
Others
|
|
Boc-Dap(Boc)-OH is an amino acid derivative with a Boc protecting group and can be used in the synthesis of primary amides .
|
-

-
- HY-D0017
-
|
DNSCl
|
Fluorescent Dye
|
Others
|
|
Dansyl chloride is a reagent that produces stable blue or blue-green fluorescent sulfonamide adducts in the reaction of aliphatic and aromatic amines with primary amino groups, and is widely used for modified amino acids, protein sequencing and amino acid analysis .
|
-

-
- HY-W005828
-
|
|
PROTAC Linkers
|
Cancer
|
|
9-(Boc-amino)nonanoic Acid is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. 9-(Boc-amino)nonanoic acid can be used as a PROTAC linker in the synthesis of PROTACs. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-D0017S
-
|
DNSCl-d6
|
Biochemical Assay Reagents
|
Others
|
|
Dansyl chloride-d6 is the deuterium labeled Dansyl chloride (HY-D0017). Dansyl chloride is a reagent that produces stable blue or blue-green fluorescent sulfonamide adducts in the reaction of aliphatic and aromatic amines with primary amino groups, and is widely used for modified amino acids, protein sequencing and amino acid analysis .
|
-

-
- HY-130695
-
|
|
ADC Linker
|
Cancer
|
|
N-(Amino-PEG5)-N-bis(PEG4-acid) is a PEG derivative containing an amino group with two terminal carboxylic acids. The amino group is reactive with carboxylic acids, activated NHS esters. The terminal carboxylic acids can react with primary amine groups in the presence of activators to form a stable amide bond. N-(Amino-PEG5)-N-bis(PEG4-acid) can be useful in the development of antibody drug conjugates (ADCs) .
|
-

-
- HY-131772
-
|
|
Fluorescent Dye
|
Others
|
|
2-AHA-cAMP is an analogue of natural signal molecule cAMP and an activator of cAMP-dependent protein kinase. 2-AHA-cAMP has a free terminal primary amino group, which can be used for coupling to gels or fluorescent dyes .
|
-

-
- HY-176347S
-
-

-
- HY-W190904
-
|
|
Biochemical Assay Reagents
|
Others
|
|
2-Amino-1,3-bis(tert-butyldimethylsilanoxy)propane has a TBDMS, acid labile protecting group. TBDMS is used for the protection of alcohol groups. The primary amine is able to react with carboxylic acids, active NHS esters and other carbonyl compounds.
|
-

-
- HY-E70453
-
|
Prostate Transglutaminase; TG4; TGase 4
|
Biochemical Assay Reagents
|
Metabolic Disease
|
|
Human prostate transglutaminase (TG4; TGase 4) is an enzyme that mediates the transfer of acyl groups from glutamine residues in proteins or peptides to primary amines and belongs to the prostate transglutaminase protein family. Human prostate transglutaminase forms an ε-(γ-glutamyl)lysine bond between proteins by transferring the acyl group of a peptide-bound glutamine residue to the primary amino group of a peptide-bound lysine residue .
|
-

-
- HY-79647
-
|
N-(Fmoc-oxy)succinimide
|
Biochemical Assay Reagents
|
Others
|
|
Fmoc-OSu (N-(Fmoc-oxy)succinimide) is an acylating agent that targets amino groups (-NH2). It can selectively protect the amino groups of amino acids by covalently binding with primary or secondary amines through nucleophilic substitution reactions. Fmoc-OSu forms a stable amide bond with the amino group to avoid side reactions of the amino group in peptide synthesis. It can also be used as a fluorescent labeling reagent to react with glycosylamines for efficient labeling of N-sugar chains. Fmoc-OSu can be used as an Fmoc protection strategy in peptide synthesis, and as a fluorescent labeling and analysis method for N-sugar chains .
|
-

-
- HY-W800715
-
|
|
Biochemical Assay Reagents
|
Others
|
|
(Acid-PEG10)-Tri-(Azide-PEG10-ethoxymethyl)-methane can undergo Click Chemistry with Propargyl, BCN or DBCO reagents. Carboxylic acid (CO2H) group is reactive with primary amino groups in the presence of EDCor HATU.
|
-

-
- HY-W041856
-
|
Boc-8-aminooctanoic acid
|
PROTAC Linkers
|
Cancer
|
|
N-Boc-8-amino-octanoic acid (Boc-8-aminooctanoic acid) can be used as a PROTAC linker in the synthesis of PROTACs. N-Boc-8-amino-octanoic acid is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-D1085
-
|
|
Fluorescent Dye
|
Others
|
|
AMCA-X-SE is a coumarin derivative that generates fixed blue fluorescence and an NHS-activated ester that forms stable amide bonds with primary amine groups. It is used as a reactive dye for labeling amino groups of peptides, proteins, and oligonucleotides. Maximum excitation/emission wavelength: 354/442 nm .
|
-

-
- HY-W591965
-
|
|
Biochemical Assay Reagents
|
Others
|
|
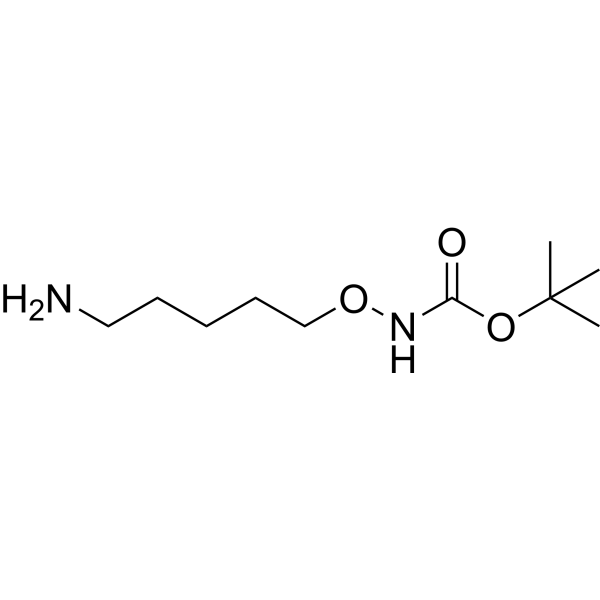
t-Boc-Aminooxy-pentane-amine is a linker containing an aminooxy group and a primary amine. The aminooxy group can be used in bioconjugation. It reacts with an aldehyde to form an oxime bond. If a reductant is used, it will form a hydroxylamine linkage. The amino group is reactive with carboxylic acids, activated NHS esters, carbonyls (ketone, aldehyde) etc.
|
-
-
- HY-W591968
-
|
|
PROTAC Linkers
|
Cancer
|
|
14-(Fmoc-amino)-tetradecanoic acid can be used as a PROTAC linker in the synthesis of PROTACs. 14-(Fmoc-amino)-tetradecanoic acid is an alkane chain with terminal Fmoc-protected amine and carboxylic acid groups. The Fmoc group can be deprotected under basic condition to obtain the free amine which can be used for further conjugations. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond.
|
-

-
- HY-W009056
-
|
|
PROTAC Linkers
|
Cancer
|
|
Fmoc-7-amino-heptanoic acid is an alkane chain with terminal Fmoc-protected amine and carboxylic acid groups. Fmoc-7-amino-heptanoic acid can be used as a PROTAC linker in the synthesis of PROTACs. The Fmoc group can be deprotected under basic condition to obtain the free amine which can be used for further conjugations. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond.
|
-

-
- HY-W190958
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Boc-NH-Tri-(carbonylethoxymethyl)-methane is a branched PEG linker with a Boc-protected amino and three terminal carboxilic acid groups. The Boc group can be deprotected under mild acidic conditions to form the free amine. The terminal carboxylic acid groups can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond.
|
-

-
- HY-W286613
-
|
|
Biochemical Assay Reagents
|
Others
|
|
N-Boc-Biocytin is a biotin PEG linker containing a carboxylic group and Boc-protected amine. Reaction of carboxylic with primary amino (-NH2) forms stable, irreversible amide bonds. The Boc group can be deprotected under acidic condition to obtain the free amine which can be used for further conjugations.
|
-

-
- HY-W800650
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Aminooxy-PEG1-amine is a chemical reagent containing an aminooxy group and a primary amine. The aminooxy group is reactive with an aldehyde to form an oxime bond. If a reductant is used, it will form a hydroxylamine linkage. The amino group is reactive withactivated NHS esters, or carboxylic acid in the presence of coupling reagent EDC. Aminooxy compounds are very reactive and sensitive; they cannot be stored for long term.
|
-

-
- HY-23629
-
|
|
PROTAC Linkers
|
Cancer
|
|
16-Aminohexadecanoic acid is an alkane chain with terminal carboxlic acid and amine groups. 16-Aminohexadecanoic acid can be used as a PROTAC linker in the synthesis of PROTACs. The amino group (NH2) is reactive with carboxylic acids, activated NHS esters, carbonyls (ketone, aldehyde) etc. The terminal carboxylic acid can react with primary amine groups of activated NHS ester to form a stable amide bond.
|
-

-
- HY-W401143
-
|
|
PROTAC Linkers
|
Cancer
|
|
15-Aminopentadecanoic Acid can be used as a PROTAC linker in the synthesis of PROTACs. 15-Aminopentadecanoic Acid is an alkane chain with terminal carboxlic acid and amine groups. The amino group (NH2) is reactive with carboxylic acids, activated NHS esters, carbonyls (ketone, aldehyde) etc. The terminal carboxylic acid can react with primary amine groups of activated NHS ester to form a stable amide bond.
|
-

-
- HY-W105727
-
|
|
PROTAC Linkers
|
Cancer
|
|
9-Aminononanoic acid can be used as a PROTAC linker and other conjugation in the synthesis of PROTACs. 9-Aminononanoic acid is an alkane chain with terminal carboxlic acid and amine groups. The amino group (NH2) is reactive with carboxylic acids, activated NHS esters, carbonyls (ketone, aldehyde) etc. The terminal carboxylic acid can react with primary amine groups of activated NHS ester to form a stable amide bond.
|
-

-
- HY-W105744
-
|
|
PROTAC Linkers
|
Cancer
|
|
10-Aminodecanoic acid is an alkane chain with terminal carboxlic acid and amine groups. 10-Aminodecanoic acid can be used as a PROTAC linker in the synthesis of PROTACs and other conjugation applications. The amino group (NH2) is reactive with carboxylic acids, activated NHS esters, carbonyls (ketone, aldehyde) etc. The terminal carboxylic acid can react with primary amine groups of activated NHS ester to form a stable amide bond.
|
-

-
- HY-W1048525B
-
|
8-Arm PEG-Amine (MW 40000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 40000) (8-Arm PEG-Amine (MW 40000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048526
-
|
8-Arm PEG-amine HCL salt (MW 5000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 5000) (8-Arm PEG-Amine (MW 5000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048526C
-
|
8-Arm PEG-amine HCL salt (MW 10000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 10000) (8-Arm PEG-Amine (MW 10000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048525C
-
|
8-Arm PEG-Amine (MW 10000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 10000) (8-Arm PEG-Amine (MW 10000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048526B
-
|
8-Arm PEG-amine HCL salt (MW 40000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 40000) (8-Arm PEG-Amine (MW 40000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048526A
-
|
8-Arm PEG-amine HCL salt (MW 20000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 20000) (8-Arm PEG-Amine (MW 20000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048525
-
|
8-Arm PEG-Amine (MW 5000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 5000) (8-Arm PEG-Amine (MW 5000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-W1048525A
-
|
8-Arm PEG-Amine (MW 20000)
|
Biochemical Assay Reagents
|
Others
|
|
8-Arm PEG-NH2 (MW 20000) (8-Arm PEG-Amine (MW 20000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-

-
- HY-D0715
-
|
Ro 20-7234
|
Fluorescent Dye
|
Others
|
|
Fluorescamine is a spirocyclic compound that is non-fluorescent. Fluorescamine reacts rapidly with primary amine groups in proteins under alkaline conditions to generate products with strong fluorescence (Ex/Em: 390/475 nm). Fluorescamine can be used to detect amine-containing compounds, including amino acids, peptides, and proteins .
|
-

-
- HY-W800813
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Carboxy-Amido-PEG5-N-Boc is a PEG linker containing a terminal carboxylic acid and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-W591982
-
|
|
Biochemical Assay Reagents
|
Others
|
|
t-Boc-N-amido-PEG12-acid is a PEG linker containing a terminal carboxylic acid and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-W591967
-
|
|
PROTAC Linkers
|
Cancer
|
|
N-Boc-15-aminopentadecanoic acid is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. N-Boc-15-aminopentadecanoic acid can be used as a PROTAC linker in the synthesis of PROTACs. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-400361
-
|
|
Biochemical Assay Reagents
|
Others
|
|
BocNH-PEG2-CH2COONHS ester is a PEG linker containing a terminal carboxylic acid and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-W089232
-
|
|
PROTAC Linkers
|
Cancer
|
|
Boc-10-Aminodecanoic acid can be used as a PROTAC linker in the synthesis of PROTACs and other conjugation applications. Boc-10-Aminodecanoic acid is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-W101723
-
|
|
PROTAC Linkers
|
Cancer
|
|
Boc-12-Ado-OH can be used as a PROTAC linker in the synthesis of PROTACs. Boc-12-Ado-OH is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-W012001
-
|
|
PROTAC Linkers
|
Cancer
|
|
Boc-7-Aminoheptanoic acid can be used as a PROTAC linker in the synthesis of PROTACs and other conjugation applications. Boc-7-Aminoheptanoic acid is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
|
-

-
- HY-D2895E
-
|
Rhodamine B-PEG-NH2 (MW 5000)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 5000) (Rhodamine B-PEG-NH2 (MW 5000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895A
-
|
Rhodamine B-PEG-NH2 (MW 600)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 600) (Rhodamine B-PEG-NH2 (MW 600)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895C
-
|
Rhodamine B-PEG-NH2 (MW 2000)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 2000) (Rhodamine B-PEG-NH2 (MW 2000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895H
-
|
Rhodamine B-PEG-NH2 (MW 10000)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 10000) (Rhodamine B-PEG-NH2 (MW 10000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895D
-
|
Rhodamine B-PEG-NH2 (MW 3400)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 3400) (Rhodamine B-PEG-NH2 (MW 3400)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895B
-
|
Rhodamine B-PEG-NH2 (MW 1000)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 1000) (Rhodamine B-PEG-NH2 (MW 1000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-D2895
-
|
Rhodamine B-PEG-NH2 (MW 400)
|
Fluorescent Dye
|
Others
|
|
RB-PEG-NH2 (MW 400) (Rhodamine B-PEG-NH2 (MW 400)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-

-
- HY-W451406B
-
|
|
Biochemical Assay Reagents
|
Others
|
|
NH2-PEG-NH2 (MW 1000) is a polyethylene glycol derivative containing two primary amine groups. The amino group can react quickly with activated carboxylic acid or carboxyl groups such as NHS esters to form stable amide bonds. The PEGylation of NH2-PEG-NH2 can increase solubility and stability, and reduce the immunogenicity of peptides and proteins, so it is mostly used to modify proteins, peptides and other substances.
|
-

-
- HY-W451406C
-
|
|
Biochemical Assay Reagents
|
Others
|
|
NH2-PEG-NH2 (MW 2000) is a polyethylene glycol derivative containing two primary amine groups. The amino group can react quickly with activated carboxylic acid or carboxyl groups such as NHS esters to form stable amide bonds. The PEGylation of NH2-PEG-NH2 can increase solubility and stability, and reduce the immunogenicity of peptides and proteins, so it is mostly used to modify proteins, peptides and other substances.
|
-

-
- HY-W451406D
-
|
|
Biochemical Assay Reagents
|
Others
|
|
NH2-PEG-NH2 (MW 10000) is a polyethylene glycol derivative containing two primary amine groups. The amino group can react quickly with activated carboxylic acid or carboxyl groups such as NHS esters to form stable amide bonds. The PEGylation of NH2-PEG-NH2 can increase solubility and stability, and reduce the immunogenicity of peptides and proteins, so it is mostly used to modify proteins, peptides and other substances.
|
-

- HY-W451406A
-
|
|
Biochemical Assay Reagents
|
Others
|
|
NH2-PEG-NH2 (MW:5000) is a polyethylene glycol derivative containing two primary amine groups. The amino group can react quickly with activated carboxylic acid or carboxyl groups such as NHS esters to form stable amide bonds. The PEGylation of NH2-PEG-NH2 can increase solubility and stability, and reduce the immunogenicity of peptides and proteins, so it is mostly used to modify proteins, peptides and other substances.
|
-

- HY-Y0623
-
|
HOSu; 1-Hydroxy-2,5-pyrrolidinedione
|
Biochemical Assay Reagents
|
Others
|
|
N-Hydroxysuccinimide (HOSu; 1-Hydroxy-2,5-pyrrolidinedione) is a covalent crosslinker commonly used in bioconjugation technology with a primary amine group. N-Hydroxysuccinimide reacts with amino groups (-NH2) to form a stable amide bond, which can modify amino-containing biomolecules. N-Hydroxysuccinimide can be used, for example, for protein labeling with fluorescent dyes and enzymes, surface activation of chromatography supports, microbeads, nanoparticles and microarray slides, and chemical synthesis of peptides. N-Hydroxysuccinimide has a wide range of applications in biomaterial synthesis (such as collagen, chitosan crosslinking), drug delivery systems (such as hydrogel preparation) and tissue engineering .
|
-

- HY-W800708
-
|
|
Biochemical Assay Reagents
|
Others
|
|
N-(TCO)-N-bis(PEG4-acid) is a branched click chemistry reagent with a terminal TCO group and two terminal carboxylic acids. This reagent can react with tetrazine-containing molecule to form a stable covalent bond . The inverse-electron demand Diels-Alder cycloaddition reaction of TCO with tetrazines is the fastest bioorthogonal reaction with exceptional selectivity. The terminal carboxylic acids can react with primary amino groups in the presence of activators (e.g. EDC, HATU ).
|
-

- HY-W190753
-
|
|
Biochemical Assay Reagents
|
Others
|
|
BocNH-PEG8-CH2CH2COONHS is a PEG linker containing an NHS ester and a Boc-protected amino group. The hydrophilic PEG spacer increases the water solubility of a compound in aqueous media. The Boc group can be deprotected under mild acidic conditions to form a free amine. The NHS ester can be used to label the primary amines (-NH2) of proteins, amine-modified oligonucleotides, and other amine-containing molecules.
|
-

- HY-D0715R
-
|
Ro 20-7234 (Standard)
|
Fluorescent Dye
|
Others
|
|
Fluorescamine (Standard) is the analytical standard of Fluorescamine (HY-D0715). This product is intended for research and analytical applications. Fluorescamine is a spirocyclic compound that is non-fluorescent. Fluorescamine reacts rapidly with primary amine groups in proteins under alkaline conditions to generate products with strong fluorescence (Ex/Em : 390/475 nm). Fluorescamine can be used to detect amine-containing compounds, including amino acids, peptides, and proteins .
|
-

- HY-W142618
-
|
|
Biochemical Assay Reagents
|
Others
|
|
D-Glucal is an organic compound belonging to the family of aldoses, which are monosaccharides containing an aldehyde functional group. It has a six-carbon structure and is derived from glucose by oxidation of the primary alcohol group at carbon 1 to an aldehyde group. D-Glucal is a white crystalline solid that is soluble in water and has a sweet taste. It is an important intermediate in the chemical synthesis of a wide variety of compounds, including pharmaceuticals, agrochemicals, and natural products. D-Glucal can be converted into other carbohydrate derivatives such as glycosides, glycoconjugates and amino sugars. It also plays a role in the study of carbohydrate chemistry, where it is used as a chiral building block for the synthesis of complex structures.
|
-

-
-
HY-L0101V
-
|
|
2,244,487 compounds
|
|
FCH Group Screening Library Collection contains about 2,244,487 lead-like compounds for biological screening. This brand new collection comprises polar molecules with pharmacologically important groups such as free carboxylic and amino groups.
|
| Cat. No. |
Product Name |
Type |
-
- HY-D0017
-
|
DNSCl
|
Protein Labeling
|
|
Dansyl chloride is a reagent that produces stable blue or blue-green fluorescent sulfonamide adducts in the reaction of aliphatic and aromatic amines with primary amino groups, and is widely used for modified amino acids, protein sequencing and amino acid analysis .
|
-
- HY-D1085
-
|
|
Fluorescent Dyes/Probes
|
|
AMCA-X-SE is a coumarin derivative that generates fixed blue fluorescence and an NHS-activated ester that forms stable amide bonds with primary amine groups. It is used as a reactive dye for labeling amino groups of peptides, proteins, and oligonucleotides. Maximum excitation/emission wavelength: 354/442 nm .
|
-
- HY-D0715
-
|
Ro 20-7234
|
Protein Labeling
|
|
Fluorescamine is a spirocyclic compound that is non-fluorescent. Fluorescamine reacts rapidly with primary amine groups in proteins under alkaline conditions to generate products with strong fluorescence (Ex/Em: 390/475 nm). Fluorescamine can be used to detect amine-containing compounds, including amino acids, peptides, and proteins .
|
-
- HY-D2895E
-
|
Rhodamine B-PEG-NH2 (MW 5000)
|
Dyes
|
|
RB-PEG-NH2 (MW 5000) (Rhodamine B-PEG-NH2 (MW 5000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895A
-
|
Rhodamine B-PEG-NH2 (MW 600)
|
Dyes
|
|
RB-PEG-NH2 (MW 600) (Rhodamine B-PEG-NH2 (MW 600)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895C
-
|
Rhodamine B-PEG-NH2 (MW 2000)
|
Dyes
|
|
RB-PEG-NH2 (MW 2000) (Rhodamine B-PEG-NH2 (MW 2000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895H
-
|
Rhodamine B-PEG-NH2 (MW 10000)
|
Dyes
|
|
RB-PEG-NH2 (MW 10000) (Rhodamine B-PEG-NH2 (MW 10000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895D
-
|
Rhodamine B-PEG-NH2 (MW 3400)
|
Dyes
|
|
RB-PEG-NH2 (MW 3400) (Rhodamine B-PEG-NH2 (MW 3400)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895B
-
|
Rhodamine B-PEG-NH2 (MW 1000)
|
Dyes
|
|
RB-PEG-NH2 (MW 1000) (Rhodamine B-PEG-NH2 (MW 1000)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D2895
-
|
Rhodamine B-PEG-NH2 (MW 400)
|
Dyes
|
|
RB-PEG-NH2 (MW 400) (Rhodamine B-PEG-NH2 (MW 400)) is a fluorescent dye composed of Rhodamine B (HY-Y0016), PEG and an amino group. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds (Ex/Em = 546/610 nm) .
|
-
- HY-D0715R
-
|
Ro 20-7234 (Standard)
|
Protein Labeling
|
|
Fluorescamine (Standard) is the analytical standard of Fluorescamine (HY-D0715). This product is intended for research and analytical applications. Fluorescamine is a spirocyclic compound that is non-fluorescent. Fluorescamine reacts rapidly with primary amine groups in proteins under alkaline conditions to generate products with strong fluorescence (Ex/Em : 390/475 nm). Fluorescamine can be used to detect amine-containing compounds, including amino acids, peptides, and proteins .
|
| Cat. No. |
Product Name |
Type |
-
- HY-79647
-
|
N-(Fmoc-oxy)succinimide
|
Biochemical Assay Reagents
|
|
Fmoc-OSu (N-(Fmoc-oxy)succinimide) is an acylating agent that targets amino groups (-NH2). It can selectively protect the amino groups of amino acids by covalently binding with primary or secondary amines through nucleophilic substitution reactions. Fmoc-OSu forms a stable amide bond with the amino group to avoid side reactions of the amino group in peptide synthesis. It can also be used as a fluorescent labeling reagent to react with glycosylamines for efficient labeling of N-sugar chains. Fmoc-OSu can be used as an Fmoc protection strategy in peptide synthesis, and as a fluorescent labeling and analysis method for N-sugar chains .
|
-
- HY-Y0623
-
|
HOSu; 1-Hydroxy-2,5-pyrrolidinedione
|
Biochemical Assay Reagents
|
|
N-Hydroxysuccinimide (HOSu; 1-Hydroxy-2,5-pyrrolidinedione) is a covalent crosslinker commonly used in bioconjugation technology with a primary amine group. N-Hydroxysuccinimide reacts with amino groups (-NH2) to form a stable amide bond, which can modify amino-containing biomolecules. N-Hydroxysuccinimide can be used, for example, for protein labeling with fluorescent dyes and enzymes, surface activation of chromatography supports, microbeads, nanoparticles and microarray slides, and chemical synthesis of peptides. N-Hydroxysuccinimide has a wide range of applications in biomaterial synthesis (such as collagen, chitosan crosslinking), drug delivery systems (such as hydrogel preparation) and tissue engineering .
|
-
- HY-W1048525B
-
|
8-Arm PEG-Amine (MW 40000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 40000) (8-Arm PEG-Amine (MW 40000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048526
-
|
8-Arm PEG-amine HCL salt (MW 5000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 5000) (8-Arm PEG-Amine (MW 5000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048526C
-
|
8-Arm PEG-amine HCL salt (MW 10000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 10000) (8-Arm PEG-Amine (MW 10000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048525C
-
|
8-Arm PEG-Amine (MW 10000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 10000) (8-Arm PEG-Amine (MW 10000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048526B
-
|
8-Arm PEG-amine HCL salt (MW 40000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 40000) (8-Arm PEG-Amine (MW 40000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048526A
-
|
8-Arm PEG-amine HCL salt (MW 20000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 20000) (8-Arm PEG-Amine (MW 20000)) HCL is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048525
-
|
8-Arm PEG-Amine (MW 5000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 5000) (8-Arm PEG-Amine (MW 5000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W1048525A
-
|
8-Arm PEG-Amine (MW 20000)
|
Drug Delivery
|
|
8-Arm PEG-NH2 (MW 20000) (8-Arm PEG-Amine (MW 20000)) is a multi-arm PEG derivative with amino groups at each end of the eight arms. The reactive primary amine or NH2 groups can react rapidly with activated carboxylic acids such as NHS esters to form stable amide bonds .
|
-
- HY-W142618
-
|
|
Carbohydrates
|
|
D-Glucal is an organic compound belonging to the family of aldoses, which are monosaccharides containing an aldehyde functional group. It has a six-carbon structure and is derived from glucose by oxidation of the primary alcohol group at carbon 1 to an aldehyde group. D-Glucal is a white crystalline solid that is soluble in water and has a sweet taste. It is an important intermediate in the chemical synthesis of a wide variety of compounds, including pharmaceuticals, agrochemicals, and natural products. D-Glucal can be converted into other carbohydrate derivatives such as glycosides, glycoconjugates and amino sugars. It also plays a role in the study of carbohydrate chemistry, where it is used as a chiral building block for the synthesis of complex structures.
|
| Cat. No. |
Product Name |
Target |
Research Area |
| Cat. No. |
Product Name |
Chemical Structure |
-
- HY-D0017S
-
|
|
|
Dansyl chloride-d6 is the deuterium labeled Dansyl chloride (HY-D0017). Dansyl chloride is a reagent that produces stable blue or blue-green fluorescent sulfonamide adducts in the reaction of aliphatic and aromatic amines with primary amino groups, and is widely used for modified amino acids, protein sequencing and amino acid analysis .
|
-

-
- HY-176347S
-
|
|
|
Alpha Feto Protein, Arg- 13C36, 15N4, Lys- 13C6, 15N2 is the 13C- and 15N-labeled Alpha Feto Protein.
|
-

Your information is safe with us. * Required Fields.
Inquiry Information
- Product Name:
- Cat. No.:
- Quantity:
- MCE Japan Authorized Agent:

































































