Search Result
Results for "
nucleotide analogue,
" in MedChemExpress (MCE) Product Catalog:
1
Biochemical Assay Reagents
1
Isotope-Labeled Compounds
| Cat. No. |
Product Name |
Target |
Research Areas |
Chemical Structure |
-
- HY-106048
-
|
5-Hydroxy-1H-imidazole-4-carboxamide; FF-10501
|
Nucleoside Antimetabolite/Analog
|
Others
|
|
Bredinin aglycone (5-Hydroxy-1H-imidazole-4-carboxamide) is a purine nucleotide analogue. Bredinin aglycone can be used to examine the efficiency of catalysts for the preparation of purine nucleotide analogues .
|
-

-
- HY-13640
-
|
GS-9219; VDC-1101
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
Rabacfosadine (GS-9219), a novel proagent of the nucleotide analogue PMEG, is designed as a cytotoxic agent that preferentially targets lymphoid cells.
|
-

-
- HY-100747
-
|
|
CD73
|
Cancer
|
|
PSB-12379, a nucleotide analogue, is a potent Ecto-5'-Nucleotidase (CD73) inhibitor with Kis of 9.03 nM (rat) and 2.21 nM (human) .
|
-
-
- HY-106048R
-
|
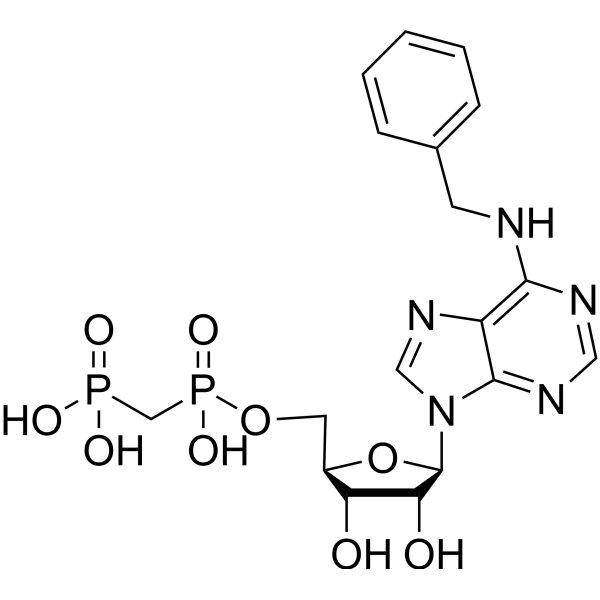
5-Hydroxy-1H-imidazole-4-carboxamide (Standard); FF-10501 (Standard)
|
Reference Standards
Nucleoside Antimetabolite/Analog
|
Others
|
|
Bredinin aglycone (Standard) is the analytical standard of Bredinin aglycone. This product is intended for research and analytical applications. Bredinin aglycone (5-Hydroxy-1H-imidazole-4-carboxamide) is a purine nucleotide analogue. Bredinin aglycone can be used to examine the efficiency of catalysts for the preparation of purine nucleotide analogues[1].
|
-

-
- HY-177164
-
-

-
- HY-177197
-
-

-
- HY-177196
-
-

-
- HY-13640A
-
|
GS-9219 succinate; VDC-1101 succinate
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
Rabacfosadine (GS-9219) succinate, a novel proagent of the nucleotide analogue PMEG, is designed as a cytotoxic agent that preferentially targets lymphoid cells .
|
-

-
- HY-100747A
-
|
|
CD73
|
Cancer
|
|
PSB-12379 disodium, a nucleotide analogue, is a potent Ecto-5'-Nucleotidase (CD73) inhibitor with Kis of 9.03 nM (rat) and 2.21 nM (human) .
|
-

-
- HY-W250161
-
|
7-Deaza-2'-dGTP
|
Telomerase
|
Cancer
|
|
7-Deaza-2′-deoxyguanosine 5′-triphosphate (7-Deaza-2'-dGTP), a nucleotide analogue, is a telomerase inhibitor (IC50: 11 μM) .
|
-

-
- HY-177198
-
-

-
- HY-100079
-
|
Mutagenic Impurity of Tenofovir Disoproxil; Tenofovir Impurity 2
|
Drug Intermediate
|
Infection
|
|
9-Propenyladenine is a mutagenic impurity in tenofovir disoproxil fumarate. Tenofovir is an antiretroviral agent known as nucleotide analogue reverse transcriptase inhibitors, which block reverse transcriptase, a crucial virus enzyme in HIV-1 and HBV.
|
-

-
- HY-109115
-
|
NUC-3373
|
Thymidylate Synthase
|
Cancer
|
|
Fosifloxuridine nafalbenamide (NUC-3373), a pyrimidine nucleotide analogue, is a Thymidylate synthase inhibitor. Fosifloxuridine nafalbenamide has anticancer activity. Fosifloxuridine nafalbenamide has the potential to evoke a host immune response and enhance immunoresearch .
|
-

-
- HY-134302
-
|
8-Hexylamino-cAMP
|
PKA
|
Others
|
|
8-HA-cAMP is a membrane-permeable cAMP analogue and an activator of cAMP-dependent protein kinase and PKA I. 8-HA-cAMP exerts metabolic stability towards mammalian cyclic nucleotide-responsive phosphodiesterases .
|
-

-
- HY-100079A
-
|
(Z)-Mutagenic Impurity of Tenofovir Disoproxil
|
HIV
|
Infection
|
|
(Z)-9-Propenyladenine is a mutagenic impurity in tenofovir disoproxil fumarate. Tenofovir is an antiretroviral agent known as nucleotide analogue reverse transcriptase (NtART) inhibitor, which blocks reverse transcriptase, a crucial virus enzyme in HIV-1 and HBV.
|
-

-
- HY-119691
-
|
LY582563; MCC-478
|
HBV
|
Infection
|
|
Alamifovir (LY582563; MCC-478), a purine nucleotide analogue proagent, shows potent activity against wild type and lamivudine resistant hepatitis B virus (HBV). Alamifovir has high activity against HBV replication and sustained antiviral effect .
|
-

-
- HY-134266
-
|
8-Bromoadenosine 5'-monophosphate; 8-Bromoadenylic acid
|
Endogenous Metabolite
|
Cardiovascular Disease
|
|
8-Bromo-AMP (8-Bromoadenosine 5'-monophosphate) is a membrane permeable cAMP analogue. 8-Bromo-AMP can improve the ability of the heart to recover from ischemia and reperfusion by increasing the levels of ATP, ADP, and total adenine nucleotides .
|
-

-
- HY-19851
-
|
GS-9131
|
Reverse Transcriptase
HIV
|
Cancer
|
|
Rovafovir etalafenamide (GS-9131), a proagent of the adenosine nucleotide analogue GS-9148, is an orally active nucleoside reverse transcriptase inhibitor (NRTI). Rovafovir etalafenamide is potent and active against a variety of NRTI mutants, and shows potent anti-HIV-1 activity .
|
-

-
- HY-135780
-
-

-
- HY-152535
-
|
|
HSV
|
Infection
Inflammation/Immunology
|
|
ATV041 is an orally active Ibuprofen (HY-78131) and nucleotide analogue. ATV041 improves oral pharmacokinetic (PK) profile and tissue distribution with anti-mouse hepatitis virus (MHV) activity. ATV041 reduces viral load, tissue damage and virus-induced inflammation in a dose-dependent manner .
|
-

-
- HY-100079R
-
|
Mutagenic Impurity of Tenofovir Disoproxil (Standard); Tenofovir Impurity 2 (Standard)
|
Reference Standards
HIV
|
Infection
|
|
9-Propenyladenine (Standard) is the analytical standard of 9-Propenyladenine. This product is intended for research and analytical applications. 9-Propenyladenine is a mutagenic impurity in tenofovir disoproxil fumarate. Tenofovir is an antiretroviral agent known as nucleotide analogue reverse transcriptase inhibitors, which block reverse transcriptase, a crucial virus enzyme in HIV-1 and HBV.
|
-

-
- HY-167911
-
|
GS 0504 sodium; HPMPC sodium; (S)-HPMPC sodium
|
CMV
Apoptosis
DNA/RNA Synthesis
Orthopoxvirus
Endogenous Metabolite
|
Infection
Cancer
|
|
Cidofovir sodium is an acyclic monophosphate nucleotide analogue and CMV inhibitor with antiviral activity. Cidofovir sodium inhibits cytomegalovirus (CMV) replication by selectively inhibiting viral DNA polymerase. Cidofovir sodium induces apoptosis and can be used in studies of AIDS cytomegalovirus retinitis, herpes, and cancer . Cidofovir sodium also has anti-orthopoxvirus and anti-variola activities .
|
-

-
- HY-135780A
-
-

-
- HY-17438
-
Cidofovir
Maximum Cited Publications
7 Publications Verification
GS 0504; HPMPC; (S)-HPMPC
|
CMV
Apoptosis
DNA/RNA Synthesis
Orthopoxvirus
Endogenous Metabolite
|
Infection
Cancer
|
|
Cidofovir (GS 0504) is an acyclic monophosphate nucleotide analogue and CMV inhibitor with antiviral activity. Cidofovir inhibits cytomegalovirus (CMV) replication by selectively inhibiting viral DNA polymerase. Cidofovir induces apoptosis and can be used in studies of AIDS cytomegalovirus retinitis, herpes, and cancer . Cidofovir also has anti-orthopoxvirus and anti-variola activities .
|
-

-
- HY-17438A
-
|
GS 0504 dihydrate; HPMPC dihydrate; (S)-HPMPC dihydrate
|
CMV
DNA/RNA Synthesis
Orthopoxvirus
Apoptosis
Endogenous Metabolite
|
Infection
Cancer
|
|
Cidofovir (GS 0504; HPMPC; (S)-HPMPC) dihydrate is an acyclic monophosphate nucleotide analogue and CMV inhibitor with antiviral activity. Cidofovir dihydrate inhibits cytomegalovirus (CMV) replication by selectively inhibiting viral DNA polymerase. Cidofovir dihydrate induces apoptosis and can be used in studies of AIDS cytomegalovirus retinitis, herpes, and cancer . Cidofovir dihydrate also has anti-orthopoxvirus and anti-variola activities .
|
-

-
- HY-17438R
-
|
GS 0504 (Standard); HPMPC (Standard); (S)-HPMPC (Standard)
|
Reference Standards
CMV
Apoptosis
DNA/RNA Synthesis
Orthopoxvirus
Endogenous Metabolite
|
Infection
Cancer
|
|
Cidofovir (Standard) is the analytical standard of Cidofovir. This product is intended for research and analytical applications. Cidofovir (GS 0504) is an acyclic monophosphate nucleotide analogue and CMV inhibitor with antiviral activity. Cidofovir inhibits cytomegalovirus (CMV) replication by selectively inhibiting viral DNA polymerase. Cidofovir induces apoptosis and can be used in studies of AIDS cytomegalovirus retinitis, herpes, and cancer . Cidofovir also has anti-orthopoxvirus and anti-variola activities .
|
-

-
- HY-171587
-
|
|
HCV
|
Infection
|
|
3′-Deoxy CTP is a nucleotide analogue and a mandatory chain terminator. 3′-Deoxy CTP can cause chain termination by lacking the 3′-hydroxyl group, inhibiting the RNA synthesis activity of HCV nonstructural protein (NS5B) polymerase and blocking viral replication. 3′-Deoxy CTP can be used to study the chain termination mechanism of HCV polymerase and the development of antiviral drugs .
|
-

-
- HY-150172A
-
|
ITP lithium salt; Inosine triphosphate lithium salt
|
Endogenous Metabolite
|
Metabolic Disease
|
|
Inosine-5'-triphosphate (ITP) lithium salt is a nucleotide analogue that acts on multiple G proteins. Inosine-5'-triphosphate lithium salt can bind to the α -subunit of G proteins and participate in G protein-mediated signal transduction as a substitute for GTP. Inosine-5'-triphosphate lithium salt interacts with the nucleotide-binding site of the G protein α -subunit, affecting the activity and function of G proteins. Inosine-5'-triphosphate lithium salt is used to explore the role of the G protein signaling pathway in cellular physiological and pathological processes .
|
-

-
- HY-W013706
-
|
ITP trisodium salt; Inosine triphosphate trisodium salt
|
Endogenous Metabolite
|
Metabolic Disease
|
|
Inosine-5'-triphosphate trisodium salt is a nucleotide analogue that acts on multiple G proteins and is widely used in G protein-related research. It can bind to the α -subunit of G proteins and participate in G protein-mediated signal transduction as a substitute for GTP. Its mechanism of action is to interact with the nucleotide-binding site of the G protein α -subunit, affecting the activity and function of G proteins. In the research field, it is mainly used to explore the role of the G protein signaling pathway in cellular physiological and pathological processes. For example, in HL-60 leukemia cells, its impact on G protein-mediated signal transduction can be studied .
|
-

-
- HY-145934A
-
|
|
OGT
Biochemical Assay Reagents
Glycosidase
|
Metabolic Disease
|
|
UDP-GlcNAz disodium (UDP-N-azidoacetylgalactosamine disodium) is the analogue of UDP-GlcNAc (HY-112174). UDP-GlcNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GlcNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GlcNAc (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
-

-
- HY-145934
-
|
UDP-N-azidoacetylgalactosamine disodium
|
Biochemical Assay Reagents
OGT
Glycosidase
|
Metabolic Disease
|
|
UDP-GalNAz (UDP-N-azidoacetylgalactosamine) disodium is the analogue of UDP-GalNAc (HY-114365). UDP-GalNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GalNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GalNAz (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
-

-
- HY-171587A
-
|
|
HCV
|
Infection
|
|
3′-Deoxy CTP trisodium is the sodium salt form of 3′-Deoxy CTP (HY-171587). 3′-Deoxy CTP trisodium is a nucleotide analogue and a mandatory chain terminator. 3′-Deoxy CTP trisodium can cause chain termination by lacking the 3′-hydroxyl group, inhibiting the RNA synthesis activity of HCV nonstructural protein (NS5B) polymerase and blocking viral replication. 3′-Deoxy CTP trisodium can be used to study the chain termination mechanism of HCV polymerase and the development of antiviral drugs .
|
-

-
- HY-D0186
-
|
|
Endogenous Metabolite
Thymidylate Synthase
|
Infection
|
|
2’-deoxyuridine is a brain-penetrant pyrimidines nucleotide that is associated with nervous system diseases. 2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. 2'-Deoxyuridine is a precursor in the synthesis of Edoxudine (HY-B1011) and also an analogue of 5-ethynyl-2'-deoxyuridine, EdU (HY-118411). 2’-deoxyuridine reduces microglial activation and improve oxidative stress damage by modulating glycolytic metabolism on the Aβ25-35-induced brain injury, which is promising for research of Alzheimer’s disease (AD) .
|
-

-
- HY-152448
-
|
|
Nucleoside Antimetabolite/Analog
|
Others
|
|
2’-Azido-2’-deoxycytidine is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc . 2’-Azido-2’-deoxycytidine is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups. 2’-Azido-2’-deoxycytidine is an azidonucleoside,and it should be phosphorylated twice to become an inhibitor of the nucleotide reductase.
|
-

-
- HY-W778990
-
|
|
Isotope-Labeled Compounds
Thymidylate Synthase
Endogenous Metabolite
|
Infection
|
|
2-Deoxyuridine-1,2,3,4,5- 13C5 is the 13C-labeled 2'-Deoxyuridine (HY-D0186). 2’-deoxyuridine is a brain-penetrant pyrimidines nucleotide that is associated with nervous system diseases. 2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. 2'-Deoxyuridine is a precursor in the synthesis of Edoxudine (HY-B1011) and also an analogue of 5-ethynyl-2'-deoxyuridine, EdU (HY-118411). 2’-deoxyuridine reduces microglial activation and improve oxidative stress damage by modulating glycolytic metabolism on the Aβ25-35-induced brain injury, which is promising for research of Alzheimer’s disease (AD) .
|
-

-
- HY-D0186R
-
|
|
Reference Standards
Endogenous Metabolite
Thymidylate Synthase
|
Infection
|
2'-Deoxyuridine (Standard) is the analytical standard of 2'-Deoxyuridine. This product is intended for research and analytical applications. 2’-deoxyuridine is a brain-penetrant pyrimidines nucleotide that is associated with nervous system diseases. 2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. 2'-Deoxyuridine is a precursor in the synthesis of Edoxudine (HY-B1011) and also an analogue of 5-ethynyl-2'-deoxyuridine, EdU (HY-118411). 2’-deoxyuridine reduces microglial activation and improve oxidative stress damage by modulating glycolytic metabolism on the Aβ25-35-induced brain injury, which is promising for research of Alzheimer’s disease (AD) .
In Vitro:The interaction between the 2-deoxyuridine and the column increases the duration of retention of 2-deoxyuridine .
Gradient elution with sodium acetate buffer-ACN eluent on two ZIC-HILIC homemade columns separates 2-deoxyuridine in under 9 min .
In Vivo:2'-Deoxyuridine (34.42 ng/mL, gavage, 15 min) passes the blood-brain barrier (BBB) to enter the hippocampus of mice brain .
2'-Deoxyuridine (20 mg/kg, gavage, daily for 4 weeks) improves cognition and memory loss and attenuates the damage to the hippocampus in Aβ25-35-induced mice model .
|
-

-
-
HY-L044
-
|
|
582 compounds
|
|
Nucleoside and nucleotide analogues are synthetic, chemically modified compounds that have been developed to mimic their physiological counterparts in order to exploit cellular metabolism and subsequently be incorporated into DNA and RNA to inhibit cellular division and viral replication. In addition to their incorporation into nucleic acids, nucleoside and nucleotide analogues can interact with and inhibit essential enzymes such as human and viral polymerases (that is, DNA-dependent DNA polymerases, RNA-dependent DNA polymerases or RNA-dependent RNA polymerases), kinases, ribonucleotide reductase, DNA methyltransferases, purine and pyrimidine nucleoside phosphorylase and thymidylate synthase. These actions of nucleoside and nucleotide analogues have potential therapeutic benefits — for example, in the inhibition of cancer cell growth, the inhibition of viral replication as well as other indications.
MCE offers a unique collection of 582 nucleotide compounds including nucleotide, nucleoside and their structural analogues. MCE Nucleotide Compound Library is a useful tool to discover anti-cancer and antiviral drugs for high throughput screening (HTS) and high content screening (HCS).
|
| Cat. No. |
Product Name |
Type |
-
- HY-145934A
-
|
|
Gene Sequencing and Synthesis
|
|
UDP-GlcNAz disodium (UDP-N-azidoacetylgalactosamine disodium) is the analogue of UDP-GlcNAc (HY-112174). UDP-GlcNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GlcNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GlcNAc (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
| Cat. No. |
Product Name |
Category |
Target |
Chemical Structure |
| Cat. No. |
Product Name |
Chemical Structure |
-
- HY-W778990
-
|
|
|
2-Deoxyuridine-1,2,3,4,5- 13C5 is the 13C-labeled 2'-Deoxyuridine (HY-D0186). 2’-deoxyuridine is a brain-penetrant pyrimidines nucleotide that is associated with nervous system diseases. 2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. 2'-Deoxyuridine is a precursor in the synthesis of Edoxudine (HY-B1011) and also an analogue of 5-ethynyl-2'-deoxyuridine, EdU (HY-118411). 2’-deoxyuridine reduces microglial activation and improve oxidative stress damage by modulating glycolytic metabolism on the Aβ25-35-induced brain injury, which is promising for research of Alzheimer’s disease (AD) .
|
-

| Cat. No. |
Product Name |
|
Classification |
-
- HY-145934A
-
|
|
|
Azide
|
|
UDP-GlcNAz disodium (UDP-N-azidoacetylgalactosamine disodium) is the analogue of UDP-GlcNAc (HY-112174). UDP-GlcNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GlcNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GlcNAc (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
-
- HY-145934
-
|
UDP-N-azidoacetylgalactosamine disodium
|
|
Azide
|
|
UDP-GalNAz (UDP-N-azidoacetylgalactosamine) disodium is the analogue of UDP-GalNAc (HY-114365). UDP-GalNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GalNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GalNAz (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
-
- HY-152448
-
|
|
|
Azide
|
|
2’-Azido-2’-deoxycytidine is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc . 2’-Azido-2’-deoxycytidine is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups. 2’-Azido-2’-deoxycytidine is an azidonucleoside,and it should be phosphorylated twice to become an inhibitor of the nucleotide reductase.
|
| Cat. No. |
Product Name |
|
Classification |
-
- HY-W013706
-
|
ITP trisodium salt; Inosine triphosphate trisodium salt
|
|
Nucleotide Analogs
|
|
Inosine-5'-triphosphate trisodium salt is a nucleotide analogue that acts on multiple G proteins and is widely used in G protein-related research. It can bind to the α -subunit of G proteins and participate in G protein-mediated signal transduction as a substitute for GTP. Its mechanism of action is to interact with the nucleotide-binding site of the G protein α -subunit, affecting the activity and function of G proteins. In the research field, it is mainly used to explore the role of the G protein signaling pathway in cellular physiological and pathological processes. For example, in HL-60 leukemia cells, its impact on G protein-mediated signal transduction can be studied .
|
-
- HY-145934
-
|
UDP-N-azidoacetylgalactosamine disodium
|
|
Nucleotide Analogs
|
|
UDP-GalNAz (UDP-N-azidoacetylgalactosamine) disodium is the analogue of UDP-GalNAc (HY-114365). UDP-GalNAc is the donor substrate of many N-acetylgalactosaminyltransferases, enzymes which transfer GalNAc from the nucleotide sugar to a saccharide or peptide acceptor . UDP-GalNAz (disodium) is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
|
-
- HY-152448
-
|
|
|
Nucleoside Analogs
Cytidine
|
|
2’-Azido-2’-deoxycytidine is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc . 2’-Azido-2’-deoxycytidine is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups. 2’-Azido-2’-deoxycytidine is an azidonucleoside,and it should be phosphorylated twice to become an inhibitor of the nucleotide reductase.
|
-
- HY-177164
-
|
|
|
Nucleoside Analogs
|
|
3'-NH2-TTP is a nucleotide analogue with an amino group modified at the 3' end of TTP.
|
Your information is safe with us. * Required Fields.
Inquiry Information
- Product Name:
- Cat. No.:
- Quantity:
- MCE Japan Authorized Agent:













































